Abstract
Background: Progressive Multifocal Encephalopathy (PML) is a subacute infection of the central nervous system (CNS) mediated by John Cunningham (JC) polyomavirus (PyV). The disease is frequently fatal, unless adaptive immunity to JC virus is restored. The disease typically occurs in immunosuppressed patients (e.g. HIV/AIDS). In the modern era, patients with hematological malignancies treated with rituximab and/or fludarabine or following immunosuppressive therapy for transplant or autoimmunity (e.g. multiple sclerosis (MS) or Crohn's disease) are also at risk. JC-PML can also occur in patients with genetic defects of immunity. Specific treatments do not exist and immunocompromised patients with history of cancer or genetic defects of immunity have no realistic chance for rapid recovery of their ability to fight infections. In subjects with Rituximab-related PML the case-fatality ratio is >90%. Survivors can be left with severe neurological disabilities.
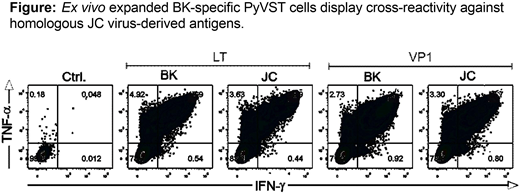
Methods: Based on our previously established procedure for generation of multi-virus virus-specific T cells using overlapping peptide libraries (pepmixes) as immunogens, we have developed dedicated polyomavirus-specific T cells (PyVST) targeting polyomavirus BK large T (LT) and Viral Protein 1 (VP1) antigens and highly cross-reactive with the structurally-homologous JC LT and VP1 proteins (see Figure). We hypothesized that adoptive transfer of donor-derived PyVSTs could be safely used for therapy of patients with refractory JC-PML. We have developed a pilot study to test the feasibility and safety of adoptive immunotherapy strategy using PyVSTs from the partially matched healthy 1st degree relative donors (sibling, parent or offspring; NIH study 16-N-0072). Adults diagnosed with PML who have no other treatment options were eligible. Patients with MS or HIV were excluded. PyVSTs were generated from donor blood leukocytes cultured for 14 days in G-rex flasks upon stimulation with BK LT and VP1 pepmixes. Subjects underwent baseline physical examination, MRI and lumbar puncture. Upon enrollment subjects received an intravenous infusion of a fixed dose of 1x10e6 (+/-10%) PyVST cells/kg. Safety monitoring period was 28 days after each infusion. Subjects were eligible for up to two additional infusions (dose 2x10e6 PyVSTs/kg) a minimum of 28 days apart if no toxicities were observed. Serial MRI and lumbar punctures were performed to monitor response. Subjects were followed for 12 months after the last infusion.
Results: Nine subjects have been enrolled and treated with at least one infusion of PyVSTs under this protocol and the trial is still accruing subjects. No immediate infusion reactions or adverse events have been observed within the safety monitoring period. No CNS immune system reconstitution syndrome (IRIS) has occurred. One subject received a single infusion and withdrew from the study following Day 14 visit due to unwillingness to travel. One subject received two doses of PyVSTs with stabilization of the disease, not requiring the 3rd infusion. This subject was withdrawn from the study just prior to the 1 year follow up visit, as he was diagnosed with the stage IV lung cancer and entered hospice. Seven subjects received 3 infusions of PyVSTs. 3 of these 7 patients died of refractory PML, all >30 days after the 3rd dose of T cells. At the time of this report one subject completed the study at one year with stable PML. The remaining subjects are in the follow-up phase of the protocol.
Conclusions: We report that partially matched T cells targeting PyVs generated from healthy related donors can be safely used for adoptive immunotherapy of severely immunocompromised patients with PML. Multiple infusions of T cells are very well tolerated at the doses of up to 2x10e6 PyVST cells/kg, displaying excellent safety profile without adverse events. Furthermore, our data suggest possible efficacy of this strategy as a life-saving therapy for patients who otherwise face a dismal prognosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal